
 |
Service
Ajinomoto CELLiST Korea基于Ajinomoto(株)的氨基酸技术和通过三十年以上积累的动物细胞培养基生产领域的经验,不仅开发了定制培养基,还成功开发了在现有培养基产品的基础上的补充剂产品, 以及提高培养基性能的微调服务,为提供适合于客户的细胞株和工艺的培养基,提供多种定制服务


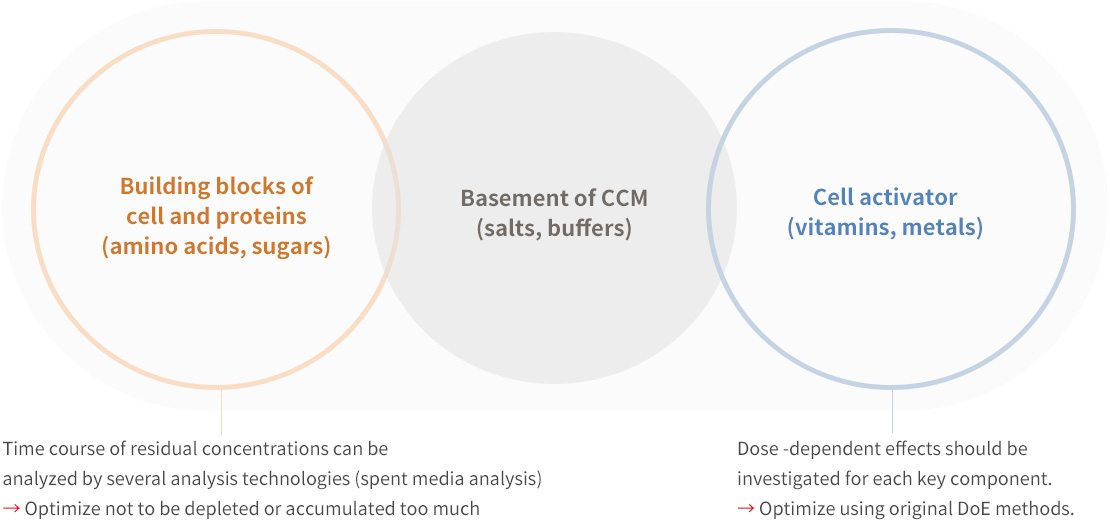
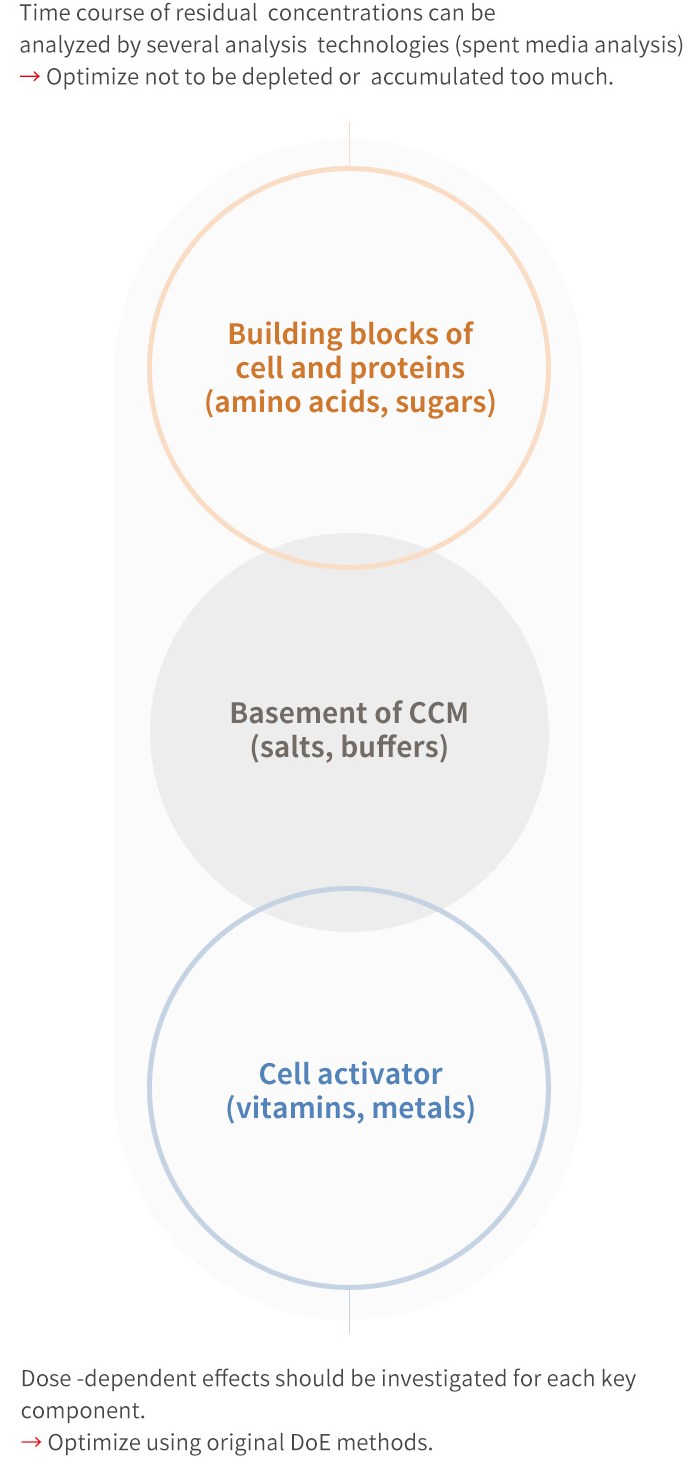
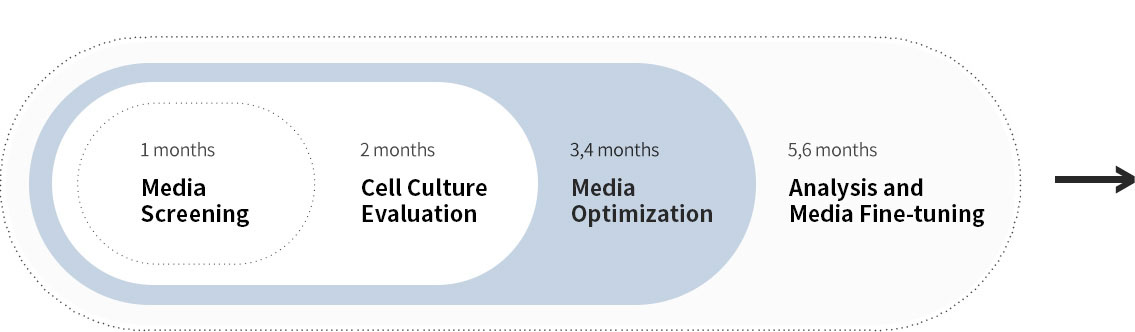
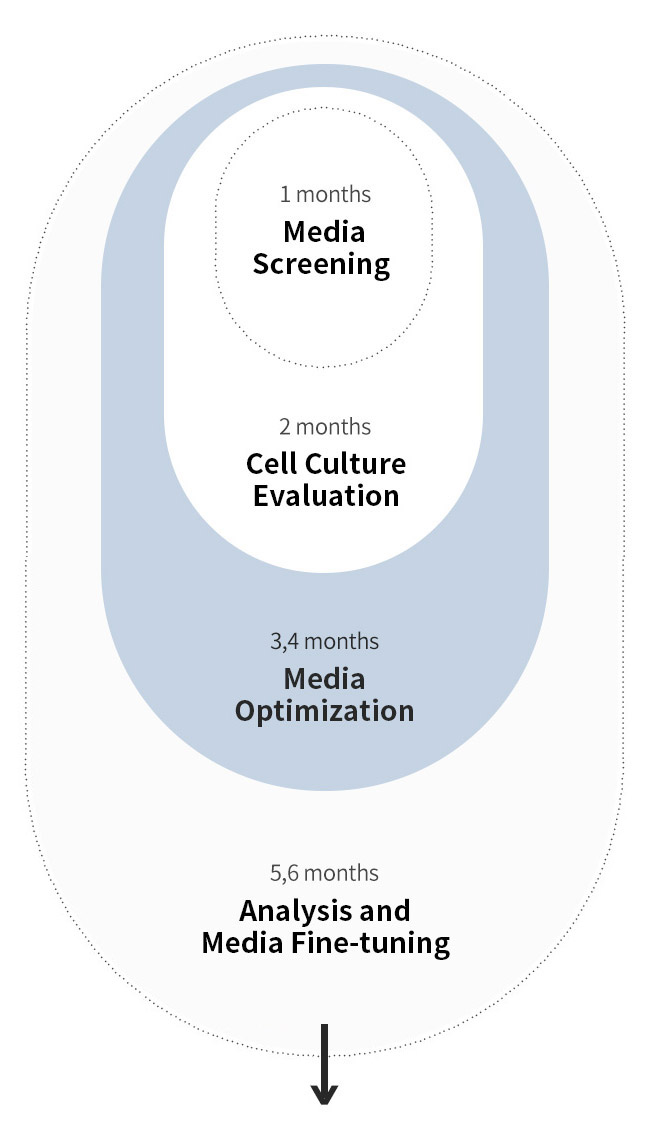
Efficient medium optimization by focusing on 3 groups of medium components
Cell culture medium consists of around 70 components, so it is unrealistic to optimize all components. We categorize medium components in to 3 groups for efficient medium optimization, the key to your success.
Offer the optimized medium under not only early development but latter/commercial phase
We provide switch program which allows customers to enhance productivity and to reduce costs while keeping product quality even under commercial phase.
| Stage | R&D | Clinical | NDA | Marketed |
|---|---|---|---|---|
| Pipelines | 27 | 21 | 1 | 4 |
Rapid analysis of a newly established CHO cell line was followed with intensive media development process, leading to significantly improved productivity in a short period of time.
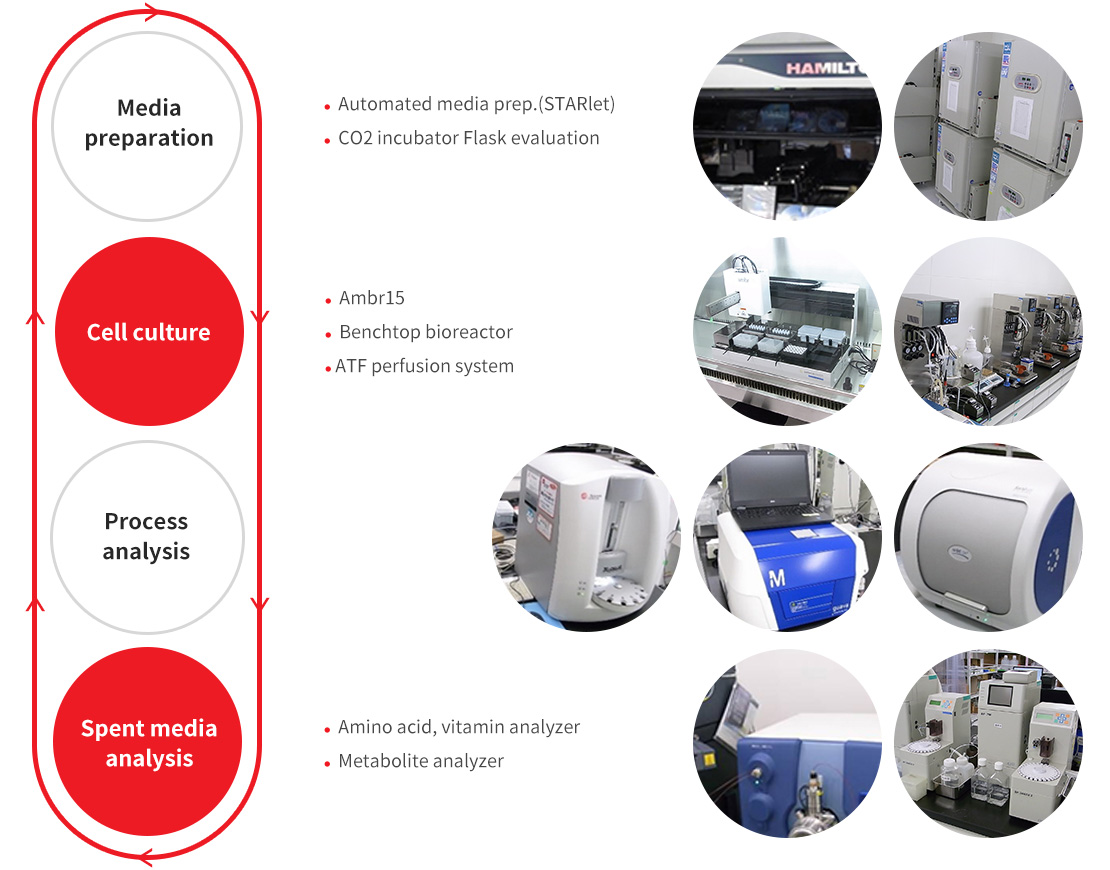
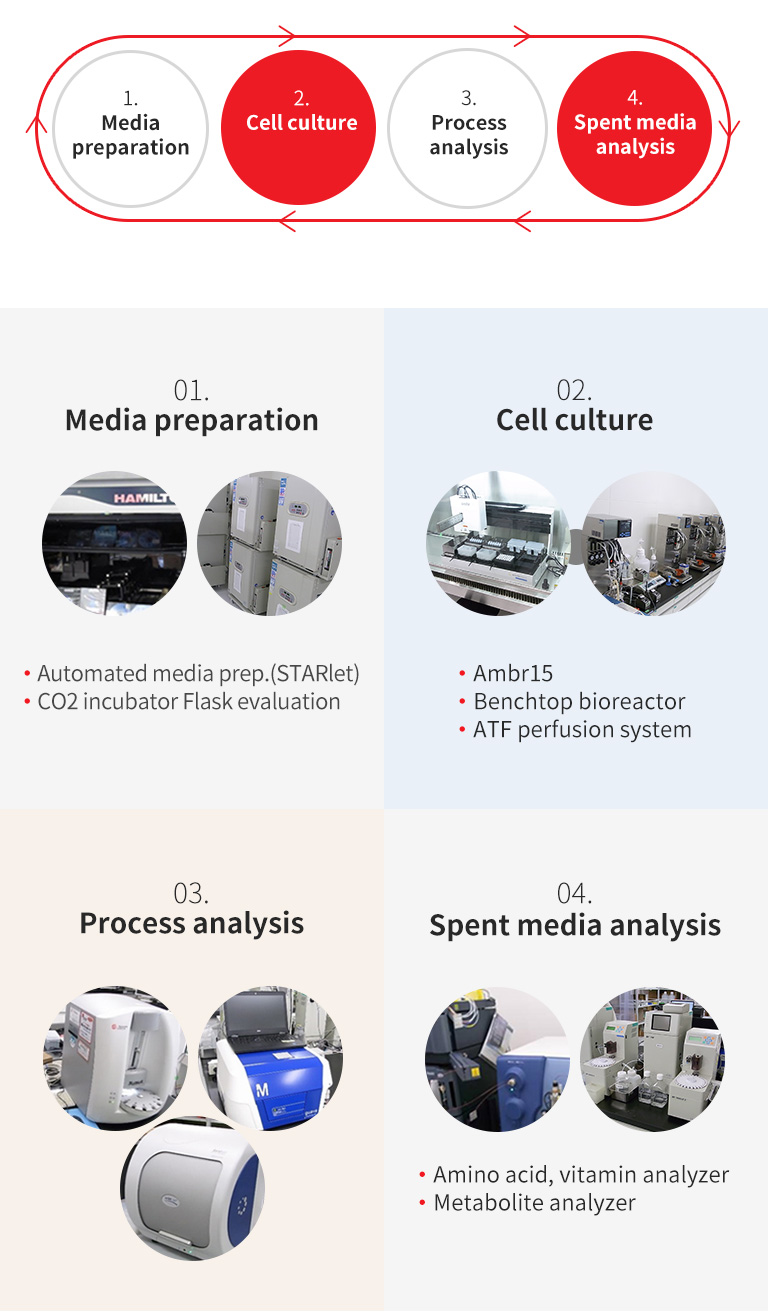
Our unique capabilities allow for strong and flexible support to the customer:
Development can be quickly initiated with starting medium optimal for the customer's cell line. Candidate redia calbe rapidly evaluated by an array of industry-leading high throughput systems supported by experienced researchers, Complete, high-throughput analysis of spent media, including amino acids, vitamins, minerals and other components, allows for a customized and efficient optimization. DoE and statistical analysis, combined with our long experience in media formulation, leads to rapid design of optimal composition. Keeping in mind customer goals and deadlines. our professional development team supports the customer throughout the process up to commercialization stage.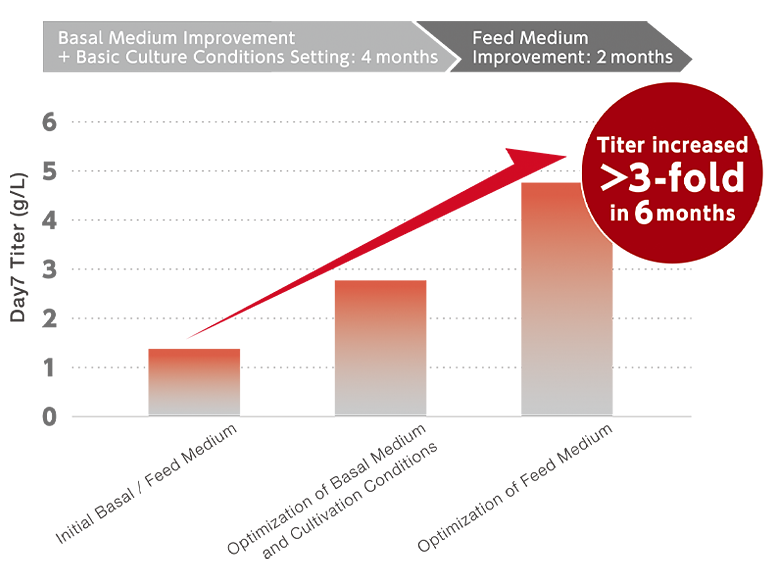
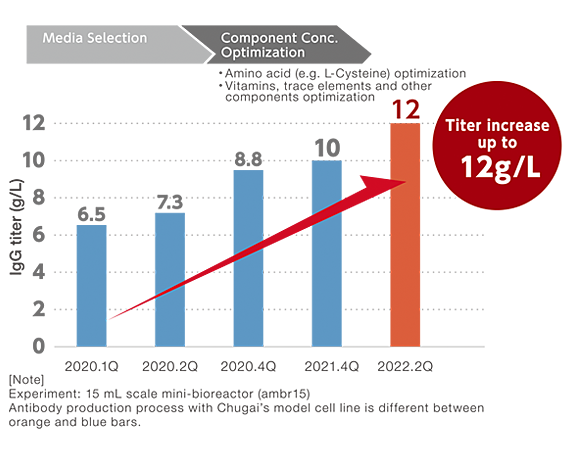
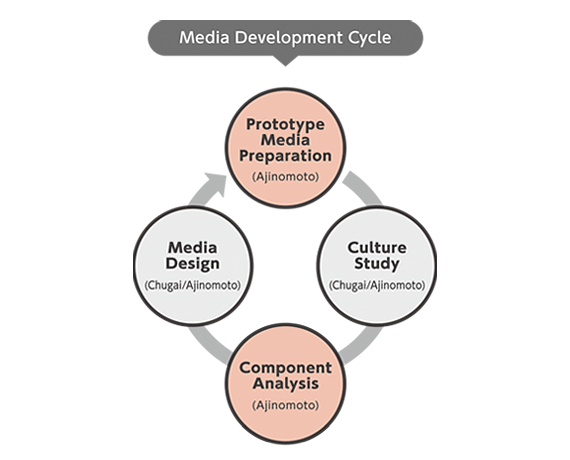
Efficient media development and optimization cycles throughout the collaboration with Chugai led to drastic increase in productivity, reaching high protein titer of 12 g/L. In the development process, our proprietary L-Cysteine technology, allowing high concentration and liquid stability, contributed to the larger improvement in titer. In addition, media performance was further enhanced based on in-house knowledge and additional spent media analysis, optimizing concentrations of potassium and phosphate, among other components.
Precipitation Inhibition

Our proprietary composition stabilized liquid media enhancing L-Cysteine.
Titer Improvement
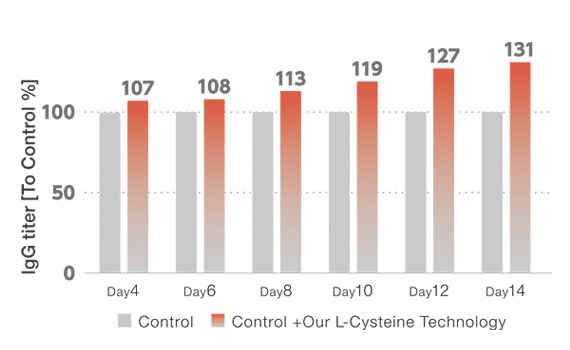
L-Cysteine high-concentration with liquid-stability increased titer.